11. The Future of Alzheimer’s Research
Over the past several years, many promising new treatments for Alzheimer’s disease have emerged from research laboratories and raised hope initially, only to be found lacking when subjected to more rigorous study. But trial and error is the nature of medical advancement. Through this process, researchers believe they are getting closer to unraveling the mysteries of the multi-faceted puzzle of Alzheimer’s disease. As fresh knowledge is gained, new ways of approaching the illness have emerged.
Researchers now pin their hopes for preventing or slowing Alzheimer’s disease on developing treatments that can be given to people before any symptoms are noticeable. Therefore, research is focused on detecting the earliest changes in the brains of people with Alzheimer’s disease and finding therapies to halt the progression. The focus has shifted from trying to cure people who already have symptoms of the disease to predicting who will get it, and trying to stop it before irreversible damage to the brain sets in.
The biological changes in the brain may begin 15 to 20 years before diminished memory or other mental dysfunction is noticeable. Therefore, it is widely believed that the best hope for treating Alzheimer’s disease will involve a therapy or combination of therapies taken in the very earliest stage of the disease—before symptoms arise. Breakthroughs in at least two areas must occur for this to become a reality. First, diagnostic tests must be developed that can accurately predict which healthy-appearing people or people with mild memory problems are actually in the early stage of Alzheimer’s disease. Second, drugs or other therapies must be developed that can be used at that early stage to stop the illness.
Advancements are occurring on both fronts. New tests are being created that detect the earliest changes.
Drugs being developed are intended to alter the course of the disease itself. Several drugs with this disease-modifying effect have not been shown to be useful when given to people who already have the memory and thinking impairments of Alzheimer’s disease. Many experts believe this is because they must be given in the very early stage before any problems with memory or thinking occur. Consequently, several studies are looking at giving drugs to people who have no symptoms of dementia but have a genetic predisposition to the disease or test positive on tests showing they are at high risk.
The failure of the new drugs to produce a dramatic improvement also points to the likelihood that no single drug alone will do the trick. Researchers are starting to believe that the multifaceted nature of Alzheimer’s disease will require a combination of treatments.
Drug Approval
Before a new drug is approved by the Food and Drug Administration it must pass through three phases of clinical trials. In Phase 1, the drug is tested in a small number of people to evaluate its safety and identify side effects. In Phase 2 trials, the treatment is given to a larger group of people to see if it works as intended. If those studies show effectiveness, the drug moves on to Phase 3, where it is tested in even larger groups of people to confirm the benefit and monitor side effects.
Many people with Alzheimer’s disease and their families choose to participate in clinical trials. There are several advantages to such participation. For example, the treatment being studied may prove effective, and you will have access to it before it becomes publicly available (see Box 11-1, “Research Studies—Should You Participate?”). for a discussion of some issues to consider before participating in a clinical trial. For information on clinical trials in your area, check the websites listed in Appendix II: Resources on page 96.
New Methods of Diagnosis
Today, the diagnosis of Alzheimer’s disease is based on symptoms, medical history, and laboratory tests. But research is underway to develop diagnostic methods that more accurately make a positive diagnosis, and do so earlier in the course of the disease. Most of the imaging and other biomarkers discussed below are not ready for routine use in clinical practice, but they represent exciting possibilities for the future.
Because Alzheimer’s disease begins to attack the brain years before the symptoms become apparent, many researchers are focused on finding “biomarkers” of Alzheimer’s disease. A biomarker is something that can be measured in the body that indicates the presence or absence of a disease, or the risk of later developing a disease. For example, cholesterol is a biomarker for cardiovascular issues. High cholesterol by itself does not cause symptoms, and people with high cholesterol do not know they have high cholesterol unless they have a blood test. But cholesterol is important because having high levels increases the risk for cardiovascular diseases.
Researchers are looking for this kind of biomarker for Alzheimer’s disease. For example, certain findings on new brain imaging techniques may serve as biomarkers. Researchers are also looking for proteins, genes, and other material present in either blood or cerebrospinal fluid (the fluid that bathes the brain and spine) that would signal the presence of the disease.
Brain imaging tests that can detect beta-amyloid or tau in the brain and tests of cerebrospinal fluid that show that beta-amyloid is present are already available. These tests can be invasive and/or expensive. They would be impractical to administer to large numbers of healthy-appearing people as screening tests to pick out those with very early Alzheimer’s disease. Therefore, there also is a push to find initial screening tests that cost less and are easier to perform to narrow down those individuals who should receive the more advanced and complex tests. Ultimately, a combination of several types of tests will most likely be needed to identify people at the earliest stage of Alzheimer’s disease.
Brain Imaging Studies
There are several ways to scan the brain to obtain an image. Positron emission tomography (PET) traces blood flow and metabolism (energy usage) in the brain. Magnetic resonance imaging (MRI) allows physicians to view a cross section of the brain and to measure the size of various brain structures. A type of MRI called functional MRI (fMRI) allows the physician to observe cross sections of the brain (and to see which areas are active) while the person is engaging in a mental activity, such as memorizing a list of words, speaking, or reading.
None of these imaging tests by themselves can detect specific changes in the brain that would allow a physician to make a definitive diagnosis of Alzheimer’s disease. But newer technologies are getting closer to that goal.
Scanning for Plaques and Tangles
Over the past several years, researchers have developed techniques that can create a direct picture of plaques and tangles in the living brain. These are called molecular imaging technologies. Just a few years ago, the only way to confirm the presence of plaques and tangles was to perform an autopsy after the person had died. But this is changing. One imaging method uses a compound that sticks to beta-amyloid plaques (the brain abnormalities that are thought to indicate the presence of Alzheimer’s disease) in the brain. This allows the clumps of beta-amyloid to be visible with PET imaging.
Three compounds with the ability to stick to beta-amyloid plaques have been approved by the FDA— florbetapir (Amyvid), flutemetamol F18 (Vizamyl), and florbetaben F18 (Neuraceq). Because these compounds have been approved for use by the FDA, they can be used by physicians to perform beta-amyloid PET brain scans outside of a research setting. However, there are important limitations regarding the usefulness of this type of scan in general practice.
If the scan shows there are no beta-amyloid plaques, then Alzheimer’s disease is probably not the cause of any symptoms of dementia. Other causes should be considered. If plaques are detected, an Alzheimer’s diagnosis would be possible. However, plaques can be present in the brains of people who don’t have Alzheimer’s disease, so other tests are needed to interpret the results of the scan.
The test has not been approved as a method to test people with normal mental function to determine if they will develop Alzheimer’s disease, although this may happen in the future. In addition, special expertise is required to interpret the scan, which may cost about $2,500 to $3,000 and is not currently covered by private insurance or Medicare. A PET scan using molecular imaging technology may be most useful for differentiating different types of dementia.
Another type of PET study detects the presence of tau, which is the other substance in the brain that distinguishes Alzheimer’s disease. This abnormal protein is responsible for the neurofibrillary tangles (described in Chapter 1) in the brains of people with Alzheimer’s disease. Several compounds that can detect tau have been developed and are being tested, but none is yet FDA approved.
PET Scans
PET imaging, without the compounds that bind to amyloid plaques or tau, is a potentially reliable method for distinguishing between different forms of dementia. For example, it may be useful for determining whether a person has Alzheimer’s disease, dementia with Lewy bodies, or frontotemporal dementia. Currently, the Centers for Medicare and Medicaid Services (CMS), the agency that oversees Medicare, will pay for PET scans, but only when doctors are unclear as to whether a patient has Alzheimer’s disease or the form of dementia called frontotemporal dementia.
MRIs
Studies with MRI—which provides a picture of the brain—have shown that this technology might be useful for early diagnosis. It appears that certain brain structures (the hippocampus and entorhinal cortex) are smaller than normal, even in the very early stages of Alzheimer’s disease. MRI can also detect thinning in the brain’s cerebral cortex. The cerebral cortex is a thin layer of tissue that covers the part of the brain called the cerebrum. It plays a key role in memory, attention, thought and language. One study found that people who were cognitively normal and found to have thinning of the cerebral cortex were at greater risk for developing Alzheimer’s disease.
A modified version of an MRI called arterial spin labeling (ASL-MRI) is being studied as a possible method to diagnose early dementia. This test is similar to an MRI, with the additional ability to look for changes in blood flow and the uptake of glucose (sugar) in the parts of the brain involved with memory. These changes can indicate altered brain function that may signal a person has dementia.
Cerebrospinal Fluid Markers
Some studies have indicated that the key biomarker may lie in cerebrospinal fluid. Cerebrospinal fluid is a clear liquid that bathes the brain and spinal cord. The brain continuously produces cerebrospinal fluid, which is then reabsorbed.
People with Alzheimer’s disease appear to have reduced levels of a type of beta-amyloid called beta-amyloid 42, as well as increased levels of tau in their cerebrospinal fluid. Therefore, it has been hypothesized that measuring levels of these substances in healthy older adults might be used to predict who will later develop Alzheimer’s disease.
Studies have shown that measuring levels of three proteins (total tau protein, phosphorylated tau, and beta-amyloid 42) in cerebrospinal fluid could predict which study participants would develop Alzheimer’s disease. One study found that the presence of a protein called soluble amyloid precursor protein beta in cerebrospinal fluid may also be predictive.
This type of testing requires a spinal tap (also called a lumbar puncture) to collect a sample of cerebrospinal fluid for testing. A spinal tap involves inserting a needle into the spinal canal in the lower back to draw out cerebrospinal fluid. It is a standard procedure used in neurology.
Neuropsychological Tests
Neuropsychological tests, done even 10 years before the appearance of Alzheimer’s symptoms, may offer clues to early warning signs.
In a study from Sweden, researchers combined the results from 47 different studies and found some specific changes in memory and cognition that, although subtle, are more common in people who later develop Alzheimer’s disease than in people who do not. The changes in cognition for people who later developed Alzheimer’s disease were not that pronounced at first, and did not differ greatly from some of the normal changes in memory that can occur in people who don’t develop the disease. But a comprehensive battery of neuropsychological tests could detect the difference between normal changes and changes that indicate Alzheimer’s disease.
Blood and Other Tests
An especially sought-after screening for early detection would be a blood test. Ideally, a simple blood test could be used to indicate whether a person is at greater-than-normal risk for Alzheimer’s disease. This could then be followed up with more advanced types of screening tests. Many attempts have been made to develop such a blood test. Several groups of researchers have found different proteins in the blood that might have this predictive ability. Tests for these proteins are not yet ready for routine use, but researchers hope that a blood test may be available in the near future.
Researchers are also looking into subtle early signs of Alzheimer’s disease that may show up before any problems with memory. For example, certain psychological and behavior changes, such apathy, anxiety, agitation, and social inappropriateness, may serve as an early warning sign. A group of researchers have described a condition called mild behavioral impairment, which may be a harbinger of dementia.
Still others are examining the usefulness of looking for characteristic changes in a person’s ability to smell (see Box 11-2, “Smell Test May Detect Dementia”) and physical changes in the eye (see Box 11-3, “Eye Scan May Show Early Sign of Dementia”) to detect Alzheimer’s disease early.
Potential Treatment Approaches
Scientists know that the brains of people with Alzheimer’s disease contain plaques and tangles (see Chapter 1). The accumulation of these substances in the brain causes brain cells to die. As more and more brain cells die, the characteristic memory and thinking problems of Alzheimer’s disease become apparent. One approach to treating the disease is to figure out what causes this process to get started, understand the cascade of events that follows the initial event, and intervene early in the process to stop it.
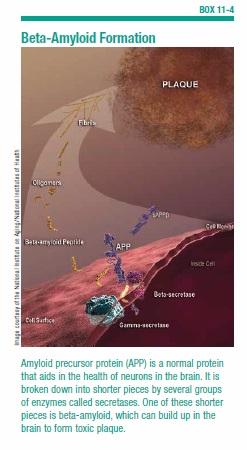
For years, the prevailing theory has been that accumulation of beta-amyloid in the brain is the trigger that sets off the chain of events that culminates in the disease. Beta-amyloid is the toxic protein that is the major component of the sticky plaques in the brains of people with Alzheimer’s disease (see Box 11-4, “Beta-Amyloid Formation”). Starting in middle age, some as-yet-unknown trigger causes beta-amyloid to start forming in just about everyone’s brain. It accumulates to a much greater degree in the brains of people with Alzheimer’s disease.
It was thought that the excessive accumulation of beta-amyloid set in motion all the other processes, like the development of tau tangles. In recent years, the contribution of tau to Alzheimer’s disease symptoms has received more attention by researchers, and a new theory has been proposed.
Because some people with accumulations of beta-amyloid plaques have been discovered to have intact memory and thinking, it’s now thought that the development of toxic tau, which results in the tangles, may be the cause of cognitive decline. According to this theory, beta-amyloid starts to accumulate in the brain at an early stage. Once abnormal tau appears, it spreads through the brain, pushing someone over the edge to developing symptoms. It’s not known what causes the tau to falter and start producing tangles.
Some researchers are looking into the role of inflammation and even infection in the brain as potential triggers of these disease processes.
Anti-Amyloid Approaches
If the accumulation of beta-amyloid is the initial trigger for the brain changes in Alzheimer’s disease, then preventing beta-amyloid from forming in the brain or clearing the beta-amyloid already there would, theoretically, prevent or halt the disease. Many drugs and other therapies based on the beta-amyloid theory have been developed and are being tested. Some work by preventing beta-amyloid from forming, and others work by removing beta-amyloid that’s already there.
Clearing Beta-Amyloid From the Brain
One approach to clearing beta-amyloid from the brain harnesses the capability of the body’s natural defenses to eliminate it. The immune system recognizes excess beta-amyloid as a threat and makes antibodies against it. Antibodies launch attacks against detrimental substances in the body. Boosting the levels of anti-amyloid antibodies has shown some promise.
Scientists have singled out the antibody against beta-amyloid and turned it into a drug therapy. Several such drugs have been developed, including solanezumab, crenezumab, and aducanumab, which bind to beta-amyloid and clear it away. These drugs showed promise in early studies with small numbers of patients. But when tested in large studies of people with mild-to-moderate Alzheimer’s disease the results were disappointing.
But the story does not end there. The drug solanezumab failed to improve mental function in people with mild-to-moderate Alzheimer’s disease in two phase 3 studies that lasted 18 months. But, when researchers looked only at those in the mild stage they saw a glimmer of hope. The drug did slow mental decline. It is now being studied in a larger number of people with mild Alzheimer’s disease. The results of that study are expected in 2017. In the meantime, the researchers extended the two phase 3 trials, offering all participants the option of taking the drug. The results of this study showed that the drug might actually be promising.
Crenezumab and aducanumab are also being studied in people with mild Alzheimer’s disease.
Several experts believe that to be effective, these drugs must be given even earlier in the course of the disease, possibly before any symptoms are apparent. Studies in people at high risk for Alzheimer’s disease but who do not yet have symptoms are underway (see “Studies in Very Early-Stage Alzheimer’s Disease,” page 90).
Prevent Beta-Amyloid from Forming
Getting rid of beta-amyloid that’s already formed in the brain is one option. Another strategy is to prevent the beta-amyloid from forming in the first place. As stated earlier, beta-amyloid is created from a larger protein called amyloid precursor protein (APP). APP is a normal protein that aids in the growth and maintenance of brain cells. The long strand of APP can be cut into smaller fragments. An enzyme acts like a pair of scissors cutting a long ribbon into shorter pieces. One of those pieces is beta-amyloid.
Scientists have identified the two enzymes that clip APP at just the right places to create beta-amyloid. They are called beta-secretase and gamma-secretase (see Box 11-4 on page 88). Researchers have also identified another enzyme called alpha-secretase. This one is important because it snips the APP right in the middle of the region where beta-amyloid would be. This makes it impossible for beta-amyloid to form.
These findings are important because a drug that blocks the snipping action of beta-secretase or gamma-secretase would prevent beta-amyloid from forming. Conversely, a drug that boosts the production of alpha-secretase could have the same effect.
Several drugs that block either beta-secretase or gamma-secretase are being studied. One of them is a drug called verubecestat, which blocks beta-secretase. Early studies of this drug showed that it reduces levels of beta-amyloid in cerebrospinal fluid. Study participants who received the drug had lower levels of beta-amyloid than those receiving placebo. Larger phase 3 studies of this drug are underway.
The future for these types of drugs is uncertain. A potential drawback of drugs that block the action of the molecular scissors gamma- and beta-secretase is that these enzymes may have other, beneficial purposes. Also, not all beta-amyloid is harmful. Scientists have found that there are different types of beta-amyloid, some of which may be beneficial. Therefore, if the action of the snipping enzymes (gamma- and beta-secretase) is blocked completely, there might be unwanted side effects.
Studies in Very Early-Stage Alzheimer’s Disease
Based on the belief that treatment for Alzheimer’s disease must be given very early, several studies are underway to test this theory and to find the right drug for effective early intervention.
One study is using the drug crenezumab in people who have the gene that guarantees they will get Alzheimer’s disease, but do not yet have any symptoms. Only about one percent of people with Alzheimer’s disease have this genetically determined form of the disease, but the results from this study may apply to the more common form of the disease. The researchers are hoping to show that by giving a drug to people who are cognitively normal now but are sure to get the disease later, it may be possible to prevent it from happening or at least to delay the onset.
The 300 participants in the study come from an extended family in Colombia, South America, and many of them carry a genetic mutation that results in dementia by age 51. Participants will receive either crenezumab or placebo, and will undergo several tests of cognitive functioning and other biomarkers of Alzheimer’s disease. The study is expected to be completed in 2017.
DIAN TU
Another study is being conducted by the Dominantly Inherited Alzheimer’s Network Trials Unit (DIAN TU) at Washington University School of Medicine. The participants in this study also carry the gene for genetically-determined Alzheimer’s disease. This study is testing two drugs targeting beta-amyloid. One is solanezumab (described earlier). The other is an antibody drug called gantenerumab, which removes beta-amyloid plaques from the brain. Results of this study are expected in 2019.
A4
The National Institutes of Health is funding a study called the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s disease (nicknamed A4). The participants in this study are not carriers of the genes for genetically determined Alzheimer’s disease. Instead, the 1,000 participants are men and women age 65 to 85 without obvious impairments in mental function as measured on tests of memory and thinking, but who have evidence of buildup of beta-amyloid plaques in the brain as measured on amyloid PET scans. This suggests that they may develop dementia. The study, which will last three years, is testing whether solanezumab prevents or delays the onset of Alzheimer’s disease.
Targeting Tau and Tangles
Many researchers are focusing their attention on neurofibrillary tangles. The tangles are caused by a protein called tau that has undergone detrimental changes. Cells use tau to construct microscopic tubes (microtubules) that allow movement of communication signals throughout the cell. In people with Alzheimer’s disease, the tau becomes misshapen and turns into disorderly twisted fibers called tangles. When this happens, the cell loses its ability to communicate with other cells, and has more difficulty taking care of and repairing itself.
Scientists are investigating the exact role of tau and tangles in the development of Alzheimer’s disease, as well as possible therapies to repair or prevent the tangles from forming.
It has been discovered that abnormal tau can spread from one neuron to neighboring neurons, thus causing a chain reaction that destroys millions of neurons. There is some evidence that the accumulation of plaques in the brain sets the stage for Alzheimer’s disease, but it’s the spread of abnormal tau that tips the scales toward Alzheimer’s disease. In a recent study, scientists conducted specialized PET scans in people with mild Alzheimer’s disease (see Box 11-5, “Tau Predicts Alzheimer’s Symptoms Better Than Beta-Amyloid”). They found both beta-amyloid deposits and tau tangles. The location of the tangles in the brain correlated with the specific problems with memory and thinking the study participants were having. This was not true for the beta-amyloid. This suggests that the presence of beta-amyloid is an early sign of Alzheimer’s disease, but it’s the spreading of the tau tangles that may be responsible for specific cognitive changes. This was a small study, and more research is needed on the role of tau in Alzheimer’s disease.
Inhaled Insulin
Another approach to treating Alzheimer’s disease involves the relationship between insulin and brain function. Insulin, which is produced in the pancreas, transports glucose (sugar) into cells, where it is used as a source of energy. Levels of insulin and glucose must be maintained at the proper balance for optimal function of cells, including brain cells. When the body does not produce enough insulin or does not properly use it, the result is diabetes.
Type 2 diabetes is caused by insulin resistance, which means the body no longer responds sufficiently to the effects of insulin. Insulin resistance starts slowly and worsens over time until a threshold level is reached, at which point diabetes is diagnosed. Insulin resistance itself (even before it becomes diabetes) can have detrimental effects. Over time, insulin resistance can cause increased inflammation and the accumulation of beta-amyloid in the brain. People with Alzheimer’s disease may have insulin resistance.
Given this possible connection, researchers hypothesized that delivering insulin to the brain may have a beneficial effect. In a small study, a group of researchers tested whether inhaled insulin would slow the progression of Alzheimer’s disease. In the study, older adults with mild cognitive impairment or mild-to-moderate Alzheimer’s disease received insulin in the form of a nasal spray or placebo for four months. Treatment with a lower dose of insulin (20 international units [IU]) improved memory, and treatment with a low (20 IU) or higher (40 IU) dose preserved general cognition and functional ability. Based on the positive findings from this study, the National Institutes of Health has funded a larger study, which is underway.
Other Approaches
Researchers are attacking the disease from many other angles in addition to those discussed above. Some scientists have come up with new theories about the cause of Alzheimer’s disease. While the amyloid hypothesis and research on tau have received much of the focus, there may be other substances in the brain worth examining. For example, a group of researchers believes that beta-amyloid oligomers are to blame rather than beta-amyloid plaques. Oligomers are floating clumps of beta-amyloid. The researchers created a genetically modified mouse that forms only oligomers and never plaques. In a study, these mice were just as cognitively impaired as their counterparts that develop plaques and oligomers.
Researchers are also looking into ways to protect brain cells and reduce inflammation in the brain.
Stem Cell Research
Curing diseases by replacing failing body systems with regenerated cells is perhaps the “Holy Grail” of scientific research. This could be possible through stem cell research. Alzheimer’s disease is just one of many conditions thought to potentially benefit from this approach. If it fulfills its promise, stem cell therapy could be used to create fresh nerve cells that would replace the ones depleted by Alzheimer’s disease.
At this point in time, the potential of stem cell research exists (early experiments have shown it to be possible for at least some diseases), but the reality of translating research into actual treatments or cures is still a long way off. Specifically, stem cell research must overcome significant hurdles.
Put simply, stem cell research is based on the fact that some cells have the flexibility to become many different types of cells. The cells of the human body are mostly quite specialized. For example, there are skin cells, liver cells, blood cells and nerve cells. Stem cells are a type of cell with the potential to become any other type of cell.
The most versatile stem cells are embryonic stem cells. Bone marrow, which is the source of many kinds of blood cells, also contains stem cells. Stem cells can also be obtained from umbilical cords. Scientists discovered that they could stimulate stem cells to become specific types of cells. This breakthrough has led to ongoing research to see if it’s possible to, for example, create insulin-producing cells for diabetics or nerve cells to replace faulty ones in the brains of people with Parkinson’s disease.
The techniques for coaxing cells into becoming a desired type are far from perfected, and there are many factors that would need to be considered in relation to Alzheimer’s, such as which types of cells would be replicated, which specific areas of the brain would be targeted, and how to get the cells to make the complicated connections found in the brain. And scientists still have to work out how they would introduce the new cells into patients and how they would ensure that the cells don’t transform into an undesirable cell type—a cancer cell, for example.
Conclusion
Research is happening at many levels, from basic research conducted in laboratories in petri dishes to new drugs and therapies being tested in humans. Scientists are simultaneously figuring out how Alzheimer’s starts and progresses, how to test for it, how to treat it, and how to prevent it. Different researchers are coming at the problem from different angles. Someday soon, one or more of these approaches may open the door to a world where Alzheimer’s is treatable.
The post 11. The Future of Alzheimer’s Research appeared first on University Health News.
Read Original Article: 11. The Future of Alzheimer’s Research »
Powered by WPeMatico