7. Treatment: Advanced Procedures
Treatment for heart failure begins with lifestyle changes and medications. When these therapies are not effective or appropriate for an individual’s specific problem, your doctor may recommend a medical procedure such as surgery.
Who Needs Surgery?
Among the people who may require surgery are those with heart valves that don’t work properly, have abnormal heart rhythms, need a medical device, or require a damaged heart to be replaced with a healthy one.
Specific procedures include systems that extract water and sodium from the body, electronic devices (pacemakers, defibrillators), mechanical circulatory support structures (ventricular assist devices, artificial heart, intra-aortic balloon pump), and surgery (coronary artery bypass grafting (CABG) and heart transplant).
According to the National Heart, Lung, and Blood Institute, CABG is the most common type of heart surgery. A half-million coronary bypass surgeries are performed each year in the U.S.
Aquapheresis
Aquapheresis (also known as ultrafiltration) is a method of extracting water from the body in a process similar to dialysis. It is sometimes used in patients admitted to the hospital with fluid overload, edema, and buildup of fluid in the abdomen from acute decompensated heart failure.
Aquapheresis can potentially remove more sodium and water than intravenous diuretics without causing kidney dysfunction or elevated potassium levels. The fact that the procedure is more expensive than loop diuretic infusions and requires large intravenous catheters prevents its widespread use.
Electronic Devices
Approximately 400,000 pacemakers and defibrillators are implanted each year in the United States. Used along with drugs, the devices can extend and improve lives.
Pacemakers
Cardiac resynchronization therapy (CRT) is a method of making the heart’s ventricles contract properly. It involves having a pacemaker implanted. Pacemakers prevent the heart from beating too slowly. They are set at a minimum number of beats per minute, and if the heart rate drops below this level, the pacemaker sends electrical impulses that stimulate the heart to beat faster.
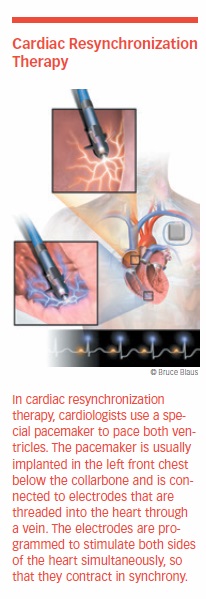
A biventricular pacemaker may be used for people with heart failure. The device is usually implanted in the left front chest, below the collarbone. Three electrodes are threaded into the heart through a vein. One is positioned in the right atrium, one in the right ventricle, and one in the left cardiac vein. The electrodes are programmed to stimulate both sides of the heart to contract at the same time. The frequency and timing are adjusted to coordinate the contraction of the atria and ventricles to produce a more efficient heartbeat.
The procedure improves cardiac function, causing less need for diuretics. CRT also improves heart failure symptoms and quality of life. It reduces complications and the risk of sudden death. It also may improve left ventricular function and reverse remodeling in some patients.
CRT is an accepted treatment for patients with moderate-to-severe heart failure who fall under New York Heart Association (NYHA) classes III and IV. The Heart Failure Society includes CRT in its guidelines, citing consistent evidence that it increases the efficiency of the heart and improves survival, morbidity, symptoms, and quality of life, regardless of symptom severity.
In patients with mild heart failure and left bundle branch block (LBBB, a problem with the heart’s electrical system), CRT with a defibrillator can reduce the likelihood of dying or suffering a nonfatal heart attack. However, CRT has less benefit in patients without LBBB. The AHA states that CRT is not indicated for patients with diastolic heart failure.
About 30 percent of patients do not respond to biventricular pacing. Determining exactly who will and will not benefit is the focus of considerable research. Scar tissue and heart-attack damage appear to influence response.
Implantable Defibrillators (ICDs)
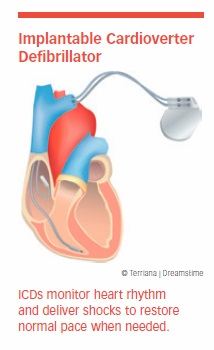
About half of all heart failure patients die suddenly from cardiac arrest. That’s why some heart failure patients receive an implantable cardioverter/defibrillator, or ICD. This device monitors the heart’s rhythm with leads implanted in the right atrium and right ventricle. When it detects the potentially deadly heart rhythm called ventricular fibrillation, it either paces the heart or delivers a shock that restores normal rhythm.
ICDs are highly effective, but the shocks can be painful. If the heart must be shocked often, an alternative (such as medication or ablation) may be advised. A less painful method of shocking the heart into rhythm involves employing five to 10 fast pacemaker pulses called a burst. In appropriately selected patients, this technique, called anti-tachycardia pacing, causes the heart rate to return to normal.
ICDs showed their superiority over medical therapy in the landmark Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). In this trial, ICDs were compared with amiodarone (Cordarone, Pacerone), the standard treatment for moderate heart failure and placebo in 2,521 patients. The ICDs were programmed to shock the heart when necessary but not to pace it. The goal was to evaluate which treatment would improve the prognosis by reducing deaths from arrhythmia.
Patients were evaluated for more than two years. Of the 829 patients who received an ICD, 259 received shocks from the device, and 68 percent of these were for life-threatening ventricular tachycardia or ventricular fibrillation. ICDs were found to reduce the risk of sudden death by 23 percent, whereas amiodarone offered no survival benefit.
As a result of the trial, the standard of care for these patients changed in favor of ICD therapy, despite a 5 percent rate of device-related complications.
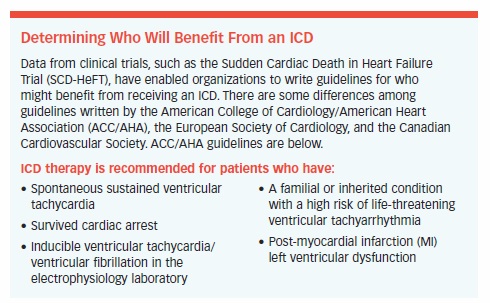
The SCD-HeFT trial held some surprises. Patients with milder cases of the disease benefited greatly from the ICD, but the device failed to help patients who had more severe cases.
ICDs also tended to work better in patients who had ejection fractions less than or equal to 30 percent, were able to walk six minutes on a treadmill, were under age 65, did not have diabetes, and were taking beta-blockers. Since exercise does not trigger shocks, patients are able to exercise, improving their overall health and quality of life.
Mechanical Support
A mechanical circulatory support (MCS) device helps the heart function when it is not working at its best. It can take over the work of your heart while you wait for a heart donor. If you are not a candidate for surgery or a heart transplant, MCSs can support your heart over a long period of time.
Ventricular Assist Devices (VADs)
When maximum medical therapy no longer controls heart failure symptoms, ventricular assist devices become an option. VADs were pioneered in the 1970s, but they were not widely used until the 1990s as a way to keep patients alive during their wait for a heart transplant. They still are used for this purpose. However, today they also are used as a permanent alternative in patients not eligible for heart transplantation.
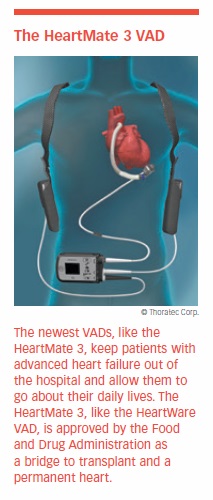
A VAD supports the heart in pumping oxygenated blood throughout the body. A battery power pack enables a VAD wearer to be out and about for several hours. This allows patients with advanced heart failure, who are generally very weak, to become more active and improve health and fitness.
Those waiting for a heart transplant are stronger and better able to withstand the stress of surgery when a donor heart becomes available. One-tenth to one-third of patients on a VAD and optimal medical therapy may recover well enough to have the device removed, even if they had been waiting for a heart transplant or are expecting to retain their VAD for life.
VADs have been called a bridge to recovery. VADs help patients feel better, have more energy, and enjoy a better quality of life.
First, Second, Third Generation VADs. First-generation VADs were designed to work like the heart, with a pulsatile chamber or sac of blood. They were powered by a cable that exited the body through the skin and was connected to a power source in a battery pack worn at the waist or carried in a shoulder bag. These early VADs were prone to infection and blood clots that caused strokes.
Second-generation VADs are totally implantable continuous-flow pumps. They push blood in one direction, like water running through a hose. The biocompatible design and new materials reduce the risk of blood clots, infections, and strokes.
The HeartMate II has been one of the most successful of these VADs. Since its approval by the FDA in 2008, the one-year survival rate with the HeartMate II has remained steady at 85 percent. For several years, the HeartMate II was used both as a bridge to transplant and as destination (final) therapy.
It has since been replaced by the HeartMate 3. The HeartMate 3 is a miniaturized third-generation VAD with a single moving part that propels blood with a rotating turbine. Suspended by magnets, the turbine doesn’t create any friction. Its design avoids the complexity of having to mimic a beating heart with four chambers.
The HeartMate 3 is biocompatible and resistant to wear and corrosion. These factors make them ideal for permanent use, especially with extended-life batteries that may be recharged using household current. The HeartMate 3 has been approved by the FDA as destination therapy.
The HeartWare HVAD, another third-generation VAD that had been approved by the FDA, was recalled by its manufacturer in June 2018 due to “unintended intermittent electrical disconnection between the power source and the controller.”
The latest VADs have allowed the technology to be used in a much larger group of patients. The new devices now have success rates approaching those of heart transplantation, which remains a limited option due to the shortage of donor organs.
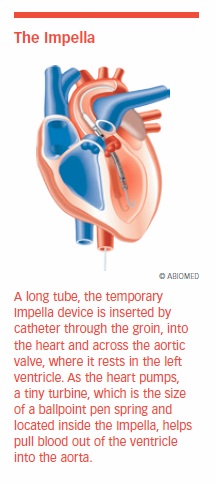
For patients with either systolic or diastolic heart failure requiring temporary support, some hospitals also use a unique device known as the Impella. The Impella is inserted by catheter through the groin, into the heart, and across the aortic valve, where it rests in the left ventricle. As the heart pumps, a tiny turbine, which is the size of a ballpoint-pen spring and located inside the Impella, helps pull blood out of the ventricle into the atrium, where it is pumped into circulation.
Total Artificial Heart
The temporary Total Artificial Heart (TAH) has been used since 1993 as a bridge to transplant and has been implanted in more patients than any other total artificial heart. In 2004, the FDA approved the TAH for temporary use in patients eligible for heart transplantation and at risk of imminent death. The TAH replaces a failing heart and is designed to restore normal blood pressure and cardiac output. This improves circulation, which enables other organs that were jeopardized due to inadequate blood supply to recover. The result is that patients are better able to withstand and recover from a heart transplant.
Intra-Aortic Balloon Pump
The Intra-Aortic Balloon Pump is a mechanical assist device inserted into the aorta through an artery in the groin. A balloon is located at the end of a catheter, and the opposite end is connected to a computer console. The balloon pump is programmed to inflate and deflate in sync with the patient’s heartbeat.
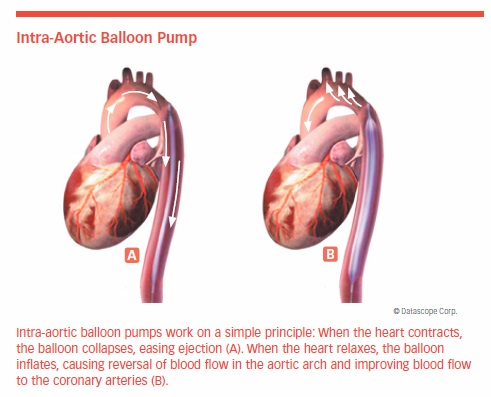
When the heart contracts, the balloon collapses, easing ejection. When the heart relaxes, the balloon inflates, causing blood flow in the aortic arch to reverse and blood flow to the coronary arteries to improve. Balloon pumps are used as temporary circulatory support in people with acute decompensated heart failure and cardiogenic shock.
Surgical Procedures
Surgery may be an option for people with advanced coronary artery disease who are not helped by medications or minimally-invasive procedures.
Coronary Artery Bypass Grafting
Coronary artery disease (CAD) plays a significant role in systolic heart failure, both directly and indirectly. Even when it is not the primary cause, CAD can prevent the heart from pumping optimally. When CAD causes significant loss of blood flow to the heart muscle, coronary artery bypass grafting (CABG) is often used to improve blood flow and relieve symptoms.
The value of CABG in relieving chest pain caused by CAD has been verified, but until recently no one knew whether its benefits would outweigh its risks in patients with heart failure and poor left ventricular function.
The Surgical Treatment for Ischemic Heart Failure (STICH) trial shed light on this issue. Patients ages 53 to 68 were treated with medications alone or with medical therapy plus CABG. The data showed that CABG increased the risk of death within two years, but after two years, the number of deaths in patients on medical therapy alone became greater. Analysis of data from the study patients at 10 years showed that fewer CABG patients died from cardiovascular causes or required hospitalization for heart-related causes than those on medications alone.
Heart Valve Repair, Replacement
Open-heart surgery is generally used to repair or replace stiff or leaky valves. A large incision is made in the chest and the heart stopped for a time so that the surgeon can repair or replace the valve.
A diseased valve may be repaired using a ring to support the damaged valve, or the entire valve may be removed and replaced by an artificial valve. Artificial valves may be made of carbon-coated plastic, tissue made from animal valves or human valves taken from donors.

Newer and less invasive techniques have been developed to replace or repair heart valves. Minimally invasive procedures make smaller incisions and mean less pain afterward and shorter hospital stays.
One of those devices is the MitraClip, which is attached to the mitral valve through a catheter that is then removed, leaving a clipped valve in place with less backflow. In a study of more than 600 patients, the MitraClip device was found to be safe and effective. It reduced the rate of hospitalizations and mortality, while improving quality of life and functional capacity. The procedure gives cardiologists an important treatment option for high-risk patients with secondary (severe) mitral regurgitation.
Heart Transplantation
Heart transplantation remains the gold-standard treatment for end-stage heart failure because it has proven to be the most effective treatment. More than half of all patients who have a heart transplant survive more than 12 years. The success of this treatment is limited by an insufficient supply of donor hearts. Recent advances in medical and VAD therapy mean that needing a heart transplant is no longer inevitable.
Suitable Candidates. Heart transplantation is only considered when heart failure reaches an advanced state. An estimated 20,000 to 70,000 people per year in the United States could benefit from a heart transplant, yet in 2017 only 3,244 patients received one.
Patients are placed on the waiting list for a donor heart only after careful evaluation to ascertain their overall medical condition and determine whether an alternative therapy might be useful. Screening determines whether patients are likely to survive the operation and regain normal function. There is no official cut-off age for transplantation, but the shortage of organs makes it unlikely that most older patients will receive an available heart.
Anyone being considered for a heart transplant must be capable of following a complex medical regimen comprising a strict diet, no smoking, exercising regularly, and taking drugs to prevent the immune system from attacking the new organ. For patients deterred by the potential complications associated with lifelong immune-suppressant medications, VADs may be a better option.
The presence of other serious medical conditions excludes some patients as transplant candidates. Perhaps more than any other operation, a transplant requires thoughtful consideration of psychological issues. Compliance with post-transplant medications, emotional stability, and a supportive environment of family and friends are critical to the long-term success of the new heart.
The name of every patient approved for transplantation is placed on the computerized UNOS list of people awaiting donor hearts.
In October 2018, the allocation scheme for eligible patients was changed significantly. Seven tiers have replaced the four previously used. The new tiers identify patients from the most to least risk of dying. In addition, available organs will be shared across a larger geographic area. These changes were made to provide the greatest chance of survival for the most potential recipients.
- Status 1 and 2 primarily include critically ill patients in the ICU who have been placed on temporary, life-supporting mechanical circulatory support devices.
- Status 3 primarily includes less critically ill patients being supported on intravenous medications or mechanical support devices. Some may require hospitalization; others are stable enough to be discharged.
- Status 4, 5, and 6 includes patients who can be maintained at home with intravenous, standard oral medications and/or stable mechanical support devices.
- Status 7 remains essentially unchanged and includes patients who are temporarily unable to undergo a transplant.
Some patients wait only days for a donor heart, while others wait months or years. A study presented in November 2018 at the AHA Scientific Sessions found that the median wait time was 10.1 months for African American patients, eight months for Caucasian patients, and 7.4 months for Hispanic patients.
The Procedure. The transplant operation takes four to eight hours. Anti-rejection drugs are given before surgery while the patient is being prepared. During the operation, the patient is connected to a cardiopulmonary bypass (heart-lung) machine to take over the function of the heart and lungs.
The diseased heart is removed as soon as the donor heart arrives in the operating room. The operation must be carefully coordinated to account for the time needed to pick up and deliver the donor heart, which may come from another part of the country.
Following the operation, patients are given intravenous medication, fluids, and drugs that suppress the immune system to prevent the new organ from being rejected.
Remarkably, as early as two or three days after the transplant, patients may begin to eat and walk around. They remain in the hospital for monitoring for one to three weeks before going home.
After Surgery. Heart transplant patients require lifelong medical care. Routine evaluations are necessary to gauge the new heart’s performance and to monitor for rejection.
The biggest obstacle in heart transplantation is preventing the body from rejecting the donor heart. Powerful immunosuppressive drugs must be taken for life. These drugs can cause serious side effects, so their dosing must be carefully adjusted and monitored to get the maximum protection with the fewest side effects.
Unfortunately, a transplanted heart is at increased risk of developing CAD due to a type of chronic rejection. Complications of post-transplant CAD are the most common cause of death after the first year. Everolimus (Certican) showed promise in reducing this risk. It is widely used in Europe but has not been approved by the FDA for use in U.S. patients with CAD. However, it is approved for preventing organ rejection after kidney transplantation.
Not the Definitive Solution
Transplantation is not the solution for the widespread problem of heart failure. No one expects that the supply of donor hearts will ever match the demand, and some patients are not candidates for transplantation. If you have been told you are inoperable due to a weak heart muscle, it may be wise to obtain a second opinion at a center specializing in heart failure and heart transplantation
Some patients also are evaluated for a heart transplant before having high-risk surgery, so that a VAD can be used as a bridge to transplant or an alternative to transplantation if surgery does not improve the condition. Ask questions, do your research, and seek a second opinion if your doctor deems your condition to be serious and says that your options are limited.
Team Approach
Whether you are just beginning to experience heart failure or facing possible heart surgery, the best strategy is through team-based care.
Heart failure is a complex problem that requires care by a team of physicians who work collaboratively with patients and their caregivers. The size of the team is not as important as the skills of each caregiver. These skills should include proficiency in monitoring for the progression of heart failure, coordinating care, prescribing treatment, and educating patients and their caregivers. The team could include cardiologists, primary care physicians, cardiovascular clinical nurse specialists (CNSs), physical therapists, dietitians, and pharmacists.
Treating heart failure in general, and heart failure with reduced ejection fraction in particular, is complex because of the breadth and depth of treatments available. These include multiple medications, sophisticated devices, surgery, and lifestyle changes.
Understanding when and how to add, switch, and determine the correct dosage of all medications is a challenge for cardiologists. Patients require additional care by an electrophysiologist if a device must be implanted, monitored, or adjusted.
Heart failure patients commonly have other heart and non-heart medical problems, as well. In fact, more than 50 percent of Medicare patients with heart failure have four or more non-cardiovascular issues, and 25 percent have six or more. This raises the risk of poorly coordinated care, miscommunications, potential medication interactions, drug-disease interactions, and other problems that interfere helping patients get better.
Clinical trials have shown that heart failure patients cared for by a team have better quality of life, fewer hospitalizations, shorter lengths of stay in the hospital, and fewer deaths. These outcomes are achieved through greater adherence to guideline-directed medical therapy, effective doses of the right drugs, and earlier recognition of the signs and symptoms of heart failure.
What’s Next?
For the most part, heart failure is preventable. Chapter 8 tells you why and shows you how.
The post 7. Treatment: Advanced Procedures appeared first on University Health News.


