4. Alzheimer’s Disease
Alzheimer’s disease (AD) is turning into a true epidemic. Today, more than 5 million Americans live with the effects of this disease, according to the Alzheimer’s Association, and the numbers are climbing. By 2050, nearly 14 million people over age 65 will have developed Alzheimer’s. The cost of caring for the disease will eat up about a third of Medicare spending.
AD is the most common cause of dementia, accounting for 60 to 80 percent of all cases. Although AD usually begins after age 60, it can very rarely affect people as young as 30.
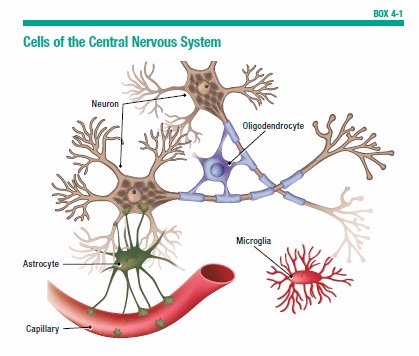
To think, learn, and remember, the brain relies on a complex communication network (see Box 4-1, “Cells of the Central Nervous System”) made up of more than 100 billion nerve cells, called neurons. These cells process and carry information throughout the brain, and send messages to direct the body’s activities. It is these nerve cells that become vulnerable in people with Alzheimer’s.
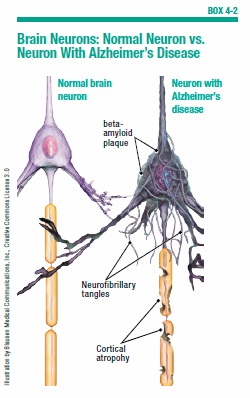
The two primary hallmarks of AD are plaques and neurofibrillary tangles. Plaques are formed by sticky clusters of a protein called beta-amyloid, which accumulate between nerve cells. Tangles are twisted clumps of another protein, called tau (see Box 4-2, “Brain Neurons: Normal Neuron vs. Neuron With Alzheimer’s Disease”). Abnormal accumulations of tau develop within the neurons as part of the Alzheimer’s disease process. Researchers believe that plaques and tangles disrupt normal brain cell activity, and block the transmission of signals between neurons, essentially shutting down communication in parts of the brain. Memory gradually deteriorates, affecting the person’s judgment and his or her ability to perform normal daily activities.
The Stages of Alzheimer’s
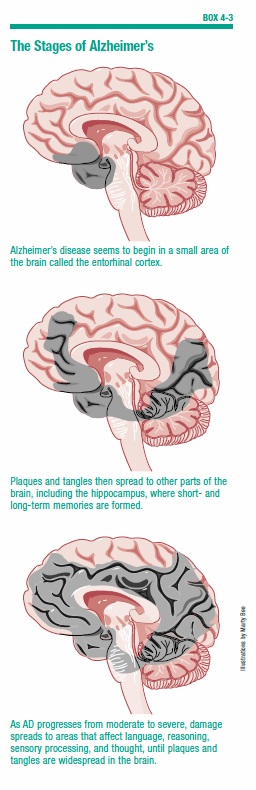
People who have Alzheimer’s typically go through a series of stages, in which symptoms gradually worsen (see Box 4-3, “The Stages of Alzheimer’s,” on page 37). However, not everyone will go through all of these stages or progress through them at the same rate.
There is some evidence that women progress to cognitive loss twice as quickly as men. Genetic or environmental causes may hasten the speed of decline in women—but these factors need to be studied further to prove any cause and effect.
Mild Cognitive Impairment (MCI)
MCI is one step beyond normal age-related memory loss, but it isn’t yet Alzheimer’s disease and it may never progress. Someone with MCI has a lot of trouble remembering simple things—for example, a familiar telephone number, or where he or she put the car keys. The person will have more trouble remembering names or words, performing complex tasks, and acquiring and retaining information. These memory lapses may become increasingly noticeable to others.
Early-Stage (mild) Alzheimer’s
A person can still be independent at this stage, but as the condition progresses, it becomes more difficult to handle multi-step tasks, such as making dinner or setting up a filing system for household bills. Family and friends, as well as the individual, might notice signs like forgetting familiar words or trouble recalling current events. The person can become increasingly withdrawn in social situations, and exhibit personality changes, irritability, anxiety, or depression. These emotional changes can put a strain on relationships with family and friends.
Mid-Stage (moderate) Alzheimer’s
This is the longest stage of AD, and it is the time when assisted care usually becomes necessary. The person will be unable to remember important information, such as his or her address or phone number. He or she will need help picking out clothes and remembering the date and time. The person may become confused or delusional at times and express irrational fears—for example, that someone is stealing from him or her.
Late-Stage (severe) Alzheimer’s
Cognitive ability becomes seriously compromised at this stage, as the person becomes unable to communicate or interact with his or her environment, and loses the ability to recognize family and friends. He or she will need help performing even the simplest tasks, such as using the bathroom or eating.
Although medications may help slow the progress, the disease is still incurable—and irreversible. However, treatments and support systems can significantly improve quality of life for people with Alzheimer’s disease.
The Search for Causes of Alzheimer’s
For many years, researchers have been searching for the Holy Grail of Alzheimer’s disease—the process underlying the disease’s development. “We are finding out that there’s a multifactorial process associated with AD,” says Gad A. Marshall, MD, a behavioral neurologist and Alzheimer’s researcher at Massachusetts General Hospital and Brigham and Women’s Hospital. Those factors include age, which clearly plays a role in the development of AD. Rare genetic mutations also come into play in a small percentage of people with the disease.
Yet researchers still don’t know exactly what factor or combination of factors sets the disease process in motion. Understanding this process could help them develop diagnostic tests to determine who is at high risk for AD so they can treat people earlier, before symptoms appear. Here are a few theories that might explain the Alzheimer’s disease process.
Amyloid Hypothesis
The amyloid hypothesis suggests that toxic beta-amyloid plaques somehow initiate the brain cell destruction observed in AD. The brain normally produces and then eliminates amyloid protein fragments. But in AD, the brain does not remove beta-amyloid proteins. Instead, this protein accumulates and forms sticky masses, or plaques, which destroy neurons and cause the gradual loss of brain tissue (see Box 4-4, “Amyloid Hypothesis: Plaques and Tangles”).
In 2014, Rudolph Tanzi, MD, director of the MGH Genetics and Aging Research unit, and his team made a significant breakthrough that strongly supports the amyloid hypothesis. For the first time, they were able to replicate the entire course of events leading to the development of Alzheimer’s disease. Their research provides the first concrete evidence that the deposit of beta-amyloid plaques in the brain can launch the process of neurological damage. “In this new system that we call ‘Alzheimer’s-in-a-dish,’ we’ve been able to show for the first time that amyloid deposition is sufficient to lead to tangles and subsequent cell death,” Dr. Tanzi said. “This new system—which can be adapted to other neurodegenerative disorders—should revolutionize drug discovery in terms of speed, costs, and physiologic relevance to disease.”
Tau Hypothesis
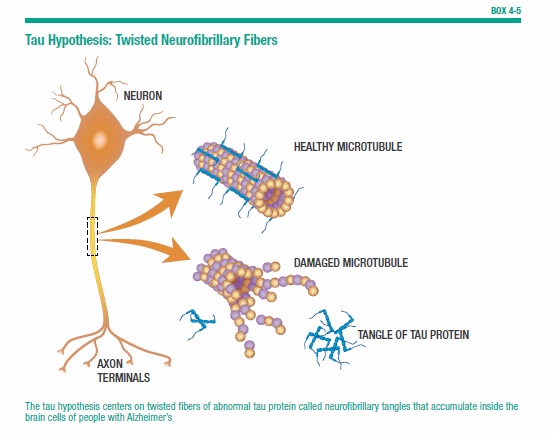
The amyloid hypothesis is a compelling explanation for the origins of Alzheimer’s, yet researchers also point to another possibility. The tau hypothesis centers on twisted fibers of abnormal tau protein called neurofibrillary tangles that accumulate inside the brain’s cells in people with Alzheimer’s. Normally, tau protein builds part of a cellular structure called a microtubule, which helps transport nutrients and other substances from one part of the nerve call to another. When tangles form, the microtubules break down, leading to the deterioration of connections to other cells and, eventually, to cell death (see Box 4-5, “Tau Hypothesis: Twisted Neurofibrillaray Fibers”).
Researchers at Mayo Clinic in Florida were able to see the process of both amyloid and tau deposition when they examined more than 3,600 postmortem brains—about 1,300 of which had confirmed Alzheimer’s. The patients were at various stages in the disease process, allowing the investigators to track the evolution of both amyloid and tau in brain tissue. The amount of tau, not amyloid, predicted the age at which people developed the disease, and the degree of cognitive loss they experienced. This suggests tau is the driver of Alzheimer’s, the researchers say.
Though the beta-amyloid vs. tau origination theories are still being debated, researchers at Massachusetts General Hospital have discovered how neurofibrillary tangles spread throughout the brain and cause memory loss. Using a mouse model of the disease, they identified a rare version of the tau protein with a high molecular weight and structure that enables it to pass easily from one neuron to the next, they reported in the journal Nature Communications. When this protein is released from one neuron, it is taken up by another neuron, moving up the neuron’s axon to be released to yet another neuron. “Our findings suggest that the release and uptake of this form of tau is an important step in the spread of disease from one brain region to another,” says Bradley Hyman, MD, PhD, director of the MGH Alzheimer’s Disease Research Center. “Since that spread likely underlies clinical progression of symptoms, targeting the mechanisms of the spreading might hold promise to stabilize disease.”
Oligomers
Oligomers.are groups of beta-amyloid that have clumped together in the brain. They’re thought to disrupt neuronal communication by changing the structure of communication points between brain cells known as synapses, and by promoting the development of neurofibrillary tangles. Many scientists now believe that beta-amyloid oligomers are more toxic to neurons than plaques. However, the exact relationship of oligomers to beta-amyloid plaques and neurofibrillary tangles is still unknown.
Tau protein, which forms the tangles in the brains of people with Alzheimer’s, also clumps together into oligomers. Increasingly, evidence suggests that tau oligomers—rather than tau protein—underlie the development of Alzheimer’s and related diseases. When researchers at the University of Texas used immunotherapy to target tau oligomers in an animal model of Alzheimer’s, the treatment both reduced levels of tau oligomers and reversed memory declines. Surprisingly, the treatment also reduced levels of beta-amyloid oligomers, suggesting the two types of oligomers work in concert to damage the brain.
Cholinergic Hypothesis
The cholinergic hypothesis was first introduced more than 30 years ago, and it’s the foundation for one of the main Alzheimer’s drug treatments. This hypothesis suggests that AD symptoms result from a decrease in production of the neurotransmitter acetylcholine. Acetylcholine is necessary for learning and memory formation. Its production is known to decline with age, but AD is associated with a much more significant decline in acetylcholine levels.
The cholinesterase inhibitor drugs that are a mainstay of AD treatment work by blocking the action of an enzyme that normally breaks down excess acetylcholine for removal, thus increasing acetylcholine levels in the brain. But while cholinesterase inhibitors can slightly improve memory and ability to carry out daily activities, they can’t slow the progression of the disease.
Excitotoxicity
In the theory of excitotoxicity, over-activation of receptors for the neurotransmitter glutamate is to blame for AD nerve damage. Glutamate is normally responsible for making neurons “fire” as they relay messages through neuronal networks, and is involved in establishing long-term memories. When normal glutamate processes are disturbed, excessive amounts of this chemical messenger build up in the space between brain cells. The excess glutamate attaches to brain cells, overstimulates them, and ultimately leads to cell death. The AD drug, memantine, works by blocking the effects of excess glutamate. But although memantine can improve symptoms of moderate to severe AD, it does not stop cognitive decline.
Excitotoxicity has been linked to AD, stroke, multiple sclerosis, and amyotrophic lateral sclerosis (ALS). However, in AD it is likely not a triggering factor. “The hypothesis that glutamate excitotoxicity specifically initiates AD would be hard to prove because it is present in so many neurological conditions,” Dr. Marshall comments. “More likely, excitotoxicity is a step along the way in the disease process.”
Oxidative Stress
Oxidative stress is another suspect on the list of possible AD causes. It occurs when unstable molecules called free radicals, generated by factors such as environmental toxins, stress, and aging, overwhelm the body’s natural antioxidant defense system. As a result, the body falls behind in its repair of cellular damage. Research suggests high levels of free radicals and oxidative stress are among the earliest changes that occur in AD. “But oxidative stress may be a marker of AD, not a cause,” says Dr. Marshall. “We don’t really know where it occurs along the chain of events in the disease process.”
Chemicals we use every day might hasten this damaging oxidative process. Repeated pesticide exposure, even at small doses, could cause problems with oxidative stress, cell dysfunction, and loss of nerve cells, which might contribute to neurodegenerative diseases such as Alzheimer’s. Two categories of pesticides in particular have been linked to Alzheimer’s—carbamates, found in insect control sprays, and organophosphates, found in herbicides and solvents. However, pesticides are just one of many possible causes. Most neurodegenerative diseases are multifactorial, triggered by a combination of environmental and genetic factors.
Leaks in the Blood-Brain Barrier
The brain is a critical and delicate organ. If dangerous substances were to easily pass through the blood vessels into the brain, the results could be disastrous. For this reason, the brain has a built-in blockade that restricts access to any dangerous foreign materials that enter the bloodstream. It’s called the blood-brain barrier. Throughout the rest of the body, blood vessels are lined with endothelial cells, which have spaces in between to allow substances to move in and out of the vessels. Yet in the brain, endothelial cells are densely packed. While essential substances like water and glucose are allowed to pass into the brain, potentially harmful substances are barred from entering.
In a 2016 study published in the journal Radiology, researchers using contrast-enhanced MRI discovered that Alzheimer’s patients had much larger leaks in the blood-brain barrier than did healthy adults of the same age. The more leakage participants had into their gray matter (the tissue where neurons are concentrated), the worse they scored on tests of cognitive function. It’s important to note that this study was small (33 participants) and preliminary, and the authors aren’t sure about the implications of their finding. Do the leaks somehow contribute to Alzheimer’s, or are they a result of it? Though there is still much to learn, this study does suggest suggests that the brain can lose the protective mechanism that shields it against damage.
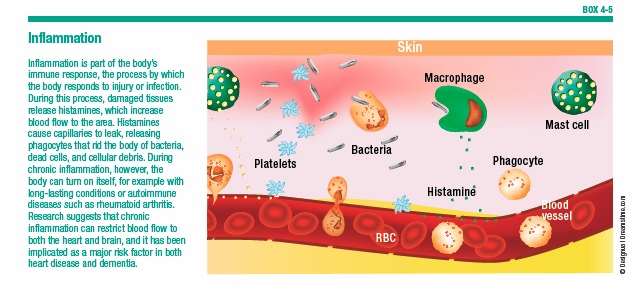
Inflammation
Inflammation has been proposed as a root cause of AD. The immune system relies on inflammation to combat viruses, cancers, and other dangerous invaders (see Box 4-5, “Inflammation”). But when inflammation persists, it can damage cells—including neurons. AD has been linked to head injury, which causes inflammation in the brain, as well as to infection, which leads to body-wide inflammation.
Having surgery might also set off the inflammatory process. Doctors have long been aware that their older patients sometimes experience cognitive decline after surgery. The challenge has been to isolate the effect of the anesthetic from the inflammation induced by the surgery itself. General anesthesia has been found to trigger memory-loss receptors in the brain. Two common anesthetic drugs—isoflurane and sevoflurane—enhance levels of beta-amyloid protein. Some research has found the memory loss can last long after surgery ends—sometimes for many months. Yet other evidence suggests the impairment is more short-term, and doesn’t leave lasting cognitive effects, especially in younger patients.
Insulin Abnormalities
Every time we eat, the hormone insulin ferries sugar from the bloodstream into the cells to be used as energy immediately or stored for later use. In people with diabetes, the body either doesn’t produce insulin, or can’t use it effectively, leading to a buildup of sugar in the bloodstream and resulting damage to organs such as the kidneys and eyes.
Researchers have discovered that insulin levels drop significantly in the early stages of AD, and progressively decline as the disease advances. Cell death and neurofibrillary tangles appear to be linked to abnormalities in insulin signaling. The theory has led to suggestions that AD may be a type of diabetes. Now, researchers are investigating whether giving insulin to people with early cognitive impairment might slow this decline. Evidence suggests it might. A nasal spray that delivers insulin directly to the brain appears to help slow or even halt the progression of neurodegeneration in people with mild cognitive impairment (MCI) or AD dementia (see Chapter 7). Further studies are needed to confirm the effectiveness and safety of insulin therapy in people with MCI and AD.
Risk Factors for Alzheimer’s
The overall risk for developing Alzheimer’s is 10 to 15 percent, but several factors can increase your odds of developing the disease. A study released in August 2015 identified nine conditions that could be responsible for up to two-thirds of Alzheimer’s cases (see Box 4-6, “Nine Factors Could Be Responsible for Most Alzheimer’s Risk,” on page 44). Many of these conditions are lifestyle-related, and therefore modifiable.
All of the factors below have been implicated in Alzheimer’s development.
Age
The single biggest risk for Alzheimer’s disease is increasing age. After you reach age 65, your risk doubles about every five years. By age 85, nearly half of all people have Alzheimer’s.
High Blood Pressure and Stroke
According to the Centers for Disease Control and Prevention (CDC), stroke is the fifth-leading cause of death in the U.S. Yet because high blood pressure rarely causes symptoms, many Americans are unaware they are at risk, and fail to seek treatment (see Box 4-7, “Treating High Blood Pressure”). Many others aren’t aware of stroke warning signs, and don’t respond to them and get medical help soon enough for treatment to be effective (see Box 4-8, “Five Signs of Stroke”). Knowing the symptoms of stroke and getting immediate medical care if you spot these signs can prevent a stroke from damaging your cognitive function. Managing risk factors that can lead to strokes can help preserve brain health as you age. These risks include high blood pressure, diabetes, high levels of “bad” LDL cholesterol, atrial fibrillation, obesity, and smoking.
Cardiovascular Disease
People with cardiovascular disease and heart conditions such as atrial fibrillation and chronic heart failure may be at significantly higher risk for cognitive impairment. Cardiovascular disease weakens the heart and disrupts the supply of oxygen-rich blood to the brain. In particular, plaques formed from LDL cholesterol can narrow the carotid arteries that carry blood up either side of the neck, slowing or blocking blood flow to the brain. This is called carotid artery disease. The greater the disruption in blood flow, the greater the decline in cognitive function, research finds. Disrupted blood flow has been linked to poorer cognitive functioning, especially in the areas of attention and executive functions, such as decision-making and thinking.
The upside is that preventing and treating heart disease and its risk factors can combat mental decline. The Framingham Study—an ongoing, long-term, multigenerational study of cardiovascular health in residents of Framingham, Massachusetts—found that better cardiovascular risk factor control led to a gradual drop in dementia cases among people age 60 and older over a three-decade period. Overall the researchers noted better management of blood pressure, higher levels of HDL (“good”) cholesterol, and declines in smoking, heart disease, and stroke.
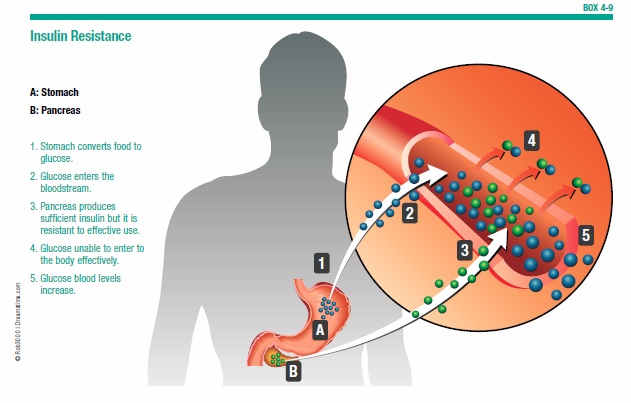
Insulin Resistance and Diabetes
About 40 percent of Americans over age 60 have insulin resistance, a condition in which the body’s cells become less sensitive to the effects of insulin and have trouble using sugar for energy as a result (see Box 4-9, “Insulin Resistance”). This condition is considered a precursor to diabetes. Research finds that high insulin levels may also be a major factor in the development of Alzheimer’s disease.
The same enzyme that breaks down insulin also breaks down the beta-amyloid protein. When insulin levels are high, the enzyme is occupied with breaking down insulin and can’t efficiently clear out beta-amyloid proteins, leading to the brain deposits that are a hallmark of Alzheimer’s disease. High blood sugar from insulin resistance is a risk factor for metabolic syndrome—a cluster of conditions that includes an enlarged waistline, high triglyceride levels, low HDL (“good”) cholesterol levels, and high blood pressure—that has been linked to memory loss in older people.
People with diabetes—especially those who are older—may be at higher risk for mild cognitive impairment, and more likely to develop dementia and Alzheimer’s disease. It is important for people with diabetes to manage the disorder with diet and medications, and to have their cognitive abilities monitored for signs of decline.
Head Injury
The brain is encased in a hard, bony shell and immersed in a bath of fluid to protect it from injury. Yet it is still vulnerable, especially when exposed to a powerful impact. A fall or other accident that jars the head can cause the brain to shift and bend inside the skull. The force of this movement can stretch nerve cells to the breaking point. These traumatic brain injuries (TBIs), which include concussions, not only lead to cognitive impairment in the area where damage occurs, but might also lead to the formation of beta-amyloid deposits in the brain. TBI has been the focus of much media and medical scrutiny in recent years, after a number of relatively young professional football players began exhibiting symptoms of cognitive decline typically found in older adults with dementia.
Although not everyone who has head trauma goes on to develop dementia, head injury can increase Alzheimer’s risk—particularly in older people. In those age 65 or older, a mild TBI could be enough to increase dementia risk, suggesting that the brain’s resiliency might diminish with age. Considering that most people hospitalized for TBI are aged 55 and older, prevention of falls and other head injuries in this population is especially important.
Depression and Alzheimer’s
Depression becomes increasingly common as we age, driven by life changes such as illness, loss of mobility, and the death of loved ones. By some estimates, depression affects more than 6.5 million seniors in the United States. Those who don’t get treated become vulnerable to all types of mental decline, including vascular dementia and Alzheimer’s disease. Symptoms of depression in older adults, which include confusion, social withdrawal, sleep problems, and delusions, can also mimic those of dementia.
Depression isn’t a normal accompaniment to aging. It’s a real medical condition that can be treated. It’s important for people of every age to recognize the warning signs—which also include feelings of hopelessness and helplessness, loss of appetite, and a lack of interest in activities they once enjoyed. Treatments such as antidepressant medicines and talk therapy can help alleviate depression.
Poor Diet and Weight Gain
A diet high in fat and low in important nutrients can set off a cascade of events that ultimately contribute to Alzheimer’s. Unhealthy diets can lead to chemical changes in the brain, accumulation of excess abdominal fat and obesity, high blood pressure, and high insulin levels, among other effects. These risk factors may, in turn, contribute to health conditions such as diabetes and stroke, which have been directly associated with increased Alzheimer’s risk. Eating a diet that promotes both heart and brain health may have a protective effect, shielding against the cumulative damage that leads to dementia.
Smoking
Although more Americans are kicking this unhealthy habit, if you’re one of the 40 million people who still smoke, you’re increasing your risk for several devastating diseases with each puff you take. Cancer, heart disease, stroke, and chronic obstructive pulmonary disease are just a few of the conditions attributed to tobacco smoke. Smoking also impairs memory function and dramatically increases your risk for dementia. Research has found that people who smoke are 50 percent more likely to develop Alzheimer’s disease or other forms of dementia than those who don’t smoke or who smoked in the past but quit. Even if you don’t smoke, you put your mind and memory at risk by spending too much time around someone who does. People who are exposed to secondhand smoke face a 44 percent increased risk of dementia.
Smoking is thought to affect memory by contributing to cardiovascular disease (a known risk factor for dementia), blood vessel damage, and hardening of the arteries. The tobacco habit also can damage brain cells and stop new cells from forming.
Mental Slowing
Leading a life lacking in mental stimulation can be detrimental to cognitive functioning, and may increase risk for dementia. Studies suggest that older adults who often engage in mentally stimulating activities, such as board or card games, puzzles, classes, social interactions, and language training are more likely to remain mentally sharp as they age.
Stress
Too much stress can accentuate memory problems, research suggests. A surge in the hormone cortisol during stressful experiences might damage synapses in the brain and contribute to memory lapses as we age.
Genetics
Genes—together with environmental factors—could help determine whether you and your siblings will develop Alzheimer’s. The more family members you have who are affected by Alzheimer’s, the greater your risk becomes.
Researchers have discovered a number of single-gene mutations that are directly associated with the early-onset (before age 60) form of Alzheimer’s, which affects fewer than 5 percent of people with the disease. Mutations in the amyloid beta A4 protein precursor (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2) genes cause the production of abnormal proteins that trigger the brain damage associated with Alzheimer’s. Investigators at Brigham and Women’s Hospital used a mouse model of Alzheimer’s to show how mutations in one of these genes—PSEN1—might damage the brain. They found that PSEN1 mutations led to a lack of gamma-secretase, an enzyme involved in generating beta-amyloid activity. Although these mutations decreased beta-amyloid in general, they increased levels of the most damaging form of the protein—beta-amyloid 42. These alterations led to memory and learning deficiencies in mice with the mutated gene.
Researchers have been unable to find specific gene mutations to blame for the late-onset form of Alzheimer’s. However, they have found a gene variant that appears to increase a person’s risk for developing the disease. Apolipoprotein E (ApoE) provides instructions for the production of a protein that helps transport cholesterol through the bloodstream and remove it from the body. About 25 percent of the population has at least one copy of ApoE4, a variation of the ApoE gene that has been associated with a higher risk for Alzheimer’s. Although ApoE4 does not cause Alzheimer’s, it may lead to brain changes that affect cognitive decline.
“It is not yet clear precisely how ApoE4 affects the brain,” says Deborah Blacker, MD, ScD, director of the Gerontology Research Unit at Massachusetts General Hospital. “But it looks like people who have the ApoE4 gene experience a more rapid loss of nerve cell functioning in the frontal lobe, which is important in maintaining higher mental functioning. It might be said that the brains of people with the ApoE4 gene age at a faster rate, and that this acceleration may increase the chances of progressing to dementia.”
ApoE may help break down sticky clusters of beta-amyloid protein that can clog the spaces between brain cells. In people with the genetic variation ApoE4, the ability to degrade beta-amyloid is impaired. This is why studies have associated the ApoE4 variation with an increased number of beta-amyloid plaques in the brain.
The first signs of this genetic variation’s effects on the brain may appear as early as in childhood. When researchers at the University of Hawaii scanned the brains of nearly 1,200 children who were APoE4 carriers, they discovered evidence of altered brain development, including smaller hippocampal volumes and poorer scores on executive function, working memory, and attention tasks. “Studying APoE4 polymorphisms in young children may provide the earliest indicators for individuals who might benefit from early interventions or preventive measures for future brain injuries and dementia,” the authors wrote in an online article published June 13, 2016, in the journal Neurology.
There is abundant evidence that people who inherit two copies of ApoE4 have a greater risk of developing Alzheimer’s than those who have just one copy. But, because not all people with Alzheimer’s have this ApoE4 genetic variation, and not all people who have the variation will develop the disease, testing for it is not currently considered a useful predictive tool.
Other Genes Identified
Other genes also appear to play a role in the development of AD. Research suggests that inherited variants of the sortilin-related receptor 1 (SORL1) gene may be involved in the abnormal production of beta-amyloid plaque in the brains of people with late-onset Alzheimer’s disease. A variant of the cell division cycle 2 gene (CDC2), has been associated with increased production of the tau protein.
The protein ubiquilin-1 controls the gathering, deposit, and degradation of several proteins associated with Alzheimer’s disease and other neurodegenerative disorders. Significantly lower ubiquilin-1 protein levels have been found in the brains of patients with late-onset AD, suggesting that its function may be diminished in Alzheimer’s disease. The logical therapeutic approach would be to increase levels of ubiquilin-1 in the affected brain regions, but more study is needed before any specific therapy can be developed.
A breakthrough international study released a few years ago yielded 11 new “susceptibility loci” (location of the genes on a chromosome) for Alzheimer’s. Researchers highlighted several previously noted pathways, including those related to amyloid and tau. But they also identified several new pathways, including ones associated with inflammation, the immune response, and lipid transport. These susceptibility loci might eventually lead to new diagnostic techniques or treatments.
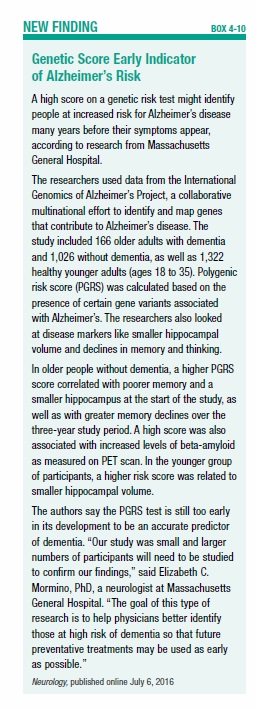
Thanks to recent advances in our understanding of Alzheimer’s susceptibility genes, researchers at Massachusetts General Hospital have developed a genetic risk score for Alzheimer’s disease. Though it’s still in the early stages, one day this test, or one like it, might identify people at high risk for the disease, long before their symptoms appear (see Box 4-10, “Genetic Score Early Indicator of Alzheimer’s Risk”).
The post 4. Alzheimer’s Disease appeared first on University Health News.
Read Original Article: 4. Alzheimer’s Disease »
Powered by WPeMatico